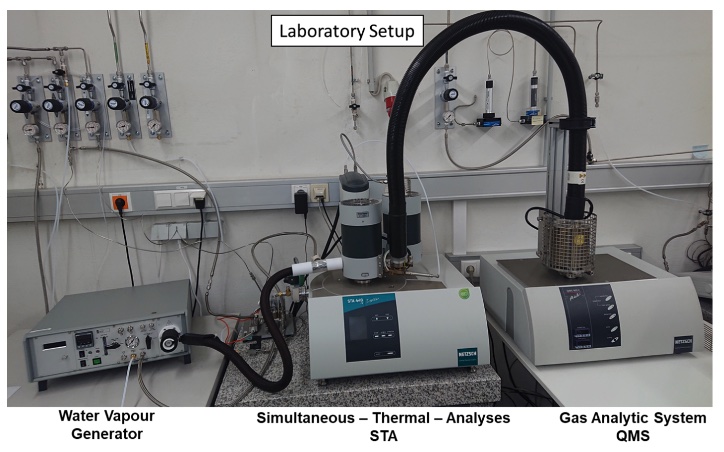
The Thermal Analysis (TA) is defined as the measurement of the change in chemical or physical properties of a sample as a function of time or temperature. The following different methods of analysis are available at the Chair of Ferrous Metallurgy:
– Differential Thermal Analysis (DTA) / Differential Scanning Calorimetry (DSC)
DTA and DSC measure, respectively, the temperature difference and the heat flow difference between a sample and a reference material (subjected to the same temperature variation in a controlled atmosphere). Properties measured by DTA / DSC include phase changes, glass transition, melting, purity, evaporation, sublimation, crystallisation, pyrolysis, heat capacity, polymerisation, denaturation / aggregation, etc.
– Thermogravimetry (TG)
Thermogravimetry is the branch of TA which examines the mass change of a sample as a function of temperature in the scanning mode or a function of time in the isothermal mode. TG is used to characterise the decomposition and thermal stability of materials under a variety of conditions, and to examine the kinetics of the physico-chemical processes occurring in the sample. Some of the material properties measured by TG include corrosion, pyrolysis, adsorption / desorption, loss of solvent, oxidation / reduction, hydration / dehydration, decomposition, etc.
– Drop shape analysis and high-temperature laser scanning confocal microscopy
These two methods of analysis characterise the optical behaviour of the material (or even the interaction of metals with refractory materials, slag and inclusions) up to the highest temperatures.